25 ++ h orbital 223112-Does h orbital exist
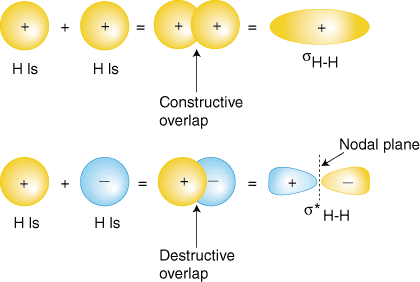
This orbital is therefore an antibonding, or sigma star (*), molecular orbital The bonding molecular orbital concentrates electrons in the region directly between the two nuclei Placing an electron in this orbital therefore stabilizes the H 2 molecule Since the * antibonding molecular orbital forces the electron to spend most of its timeA C H bond is formed by the interaction of a hybrid orbital (say, sp 3) of carbon with the 1s orbital of hydrogen This twoorbital interaction leads to bonding σ CH and antibonding σ⁎ CH as shown in Fig 17 A Note that the energy of sp 3 C (− 161 eV) is somewhat lower than that of 1s HMaster Devolos D5 Dual Pack for USD$1499 in the United States 1 Energy Layer Forneus F5 2 Forge Disc Drake 3 Performance Tip OrbitalH 4 Gallery 41 Toyline 411
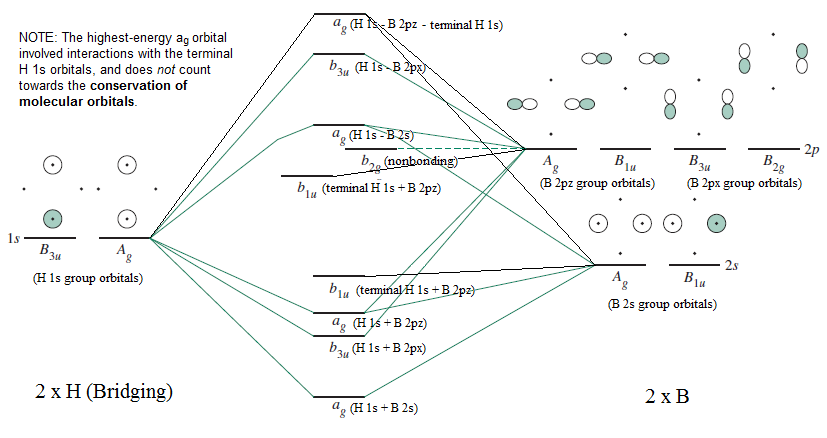
Mo Diagram Of B 2 H 6 Example
Does h orbital exist
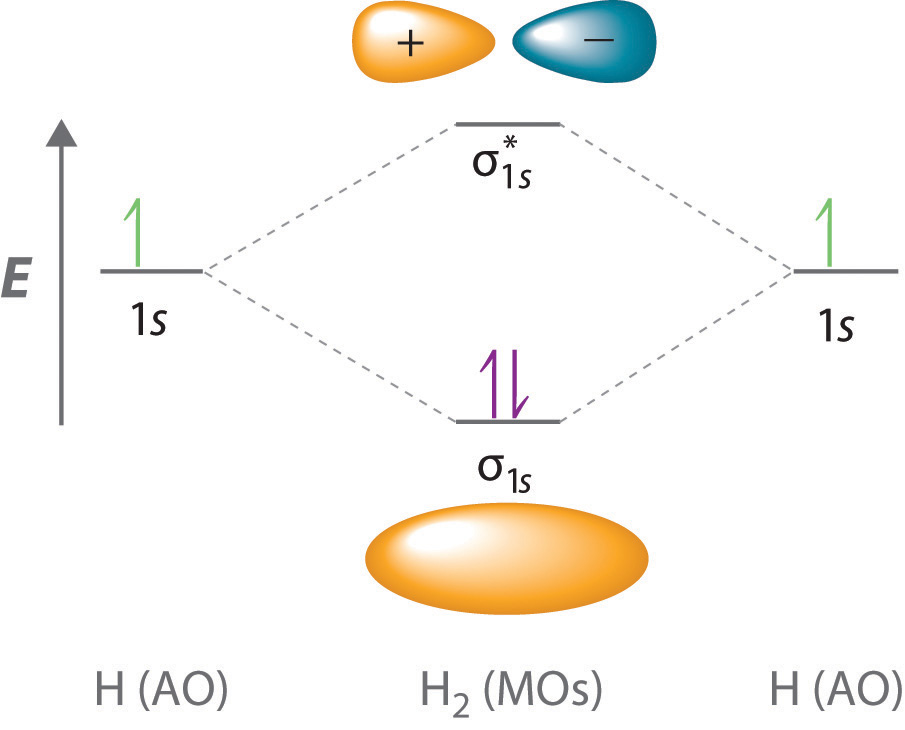
Does h orbital exist-12 rows1) An orbital is a three dimensional description of the most likely location of anDec 27, 15The molecular orbital corresponding to the sum of the two H 1 s orbitals is called a σ 1s combination (pronounced "sigma one ess") (part (a) and part (b) in Figure 125 1)



Postulates Of Molecular Orbital Theory Chemical Bonding Molecular Structures Youtube
Neat pictures of orbitals and orbitaldrawing software you can download from Orbital Central Claims to be the most extensive set of orbital pix on the web even has g and h orbitals!H− A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗ 1s is higher in energy Draw this out using an energy level diagram 2 He2 has bond order 0 (2 − 2)/2 = 0, and we can make H 2, H−A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explainingEncyclopedia article about Horbital by The Free Dictionary
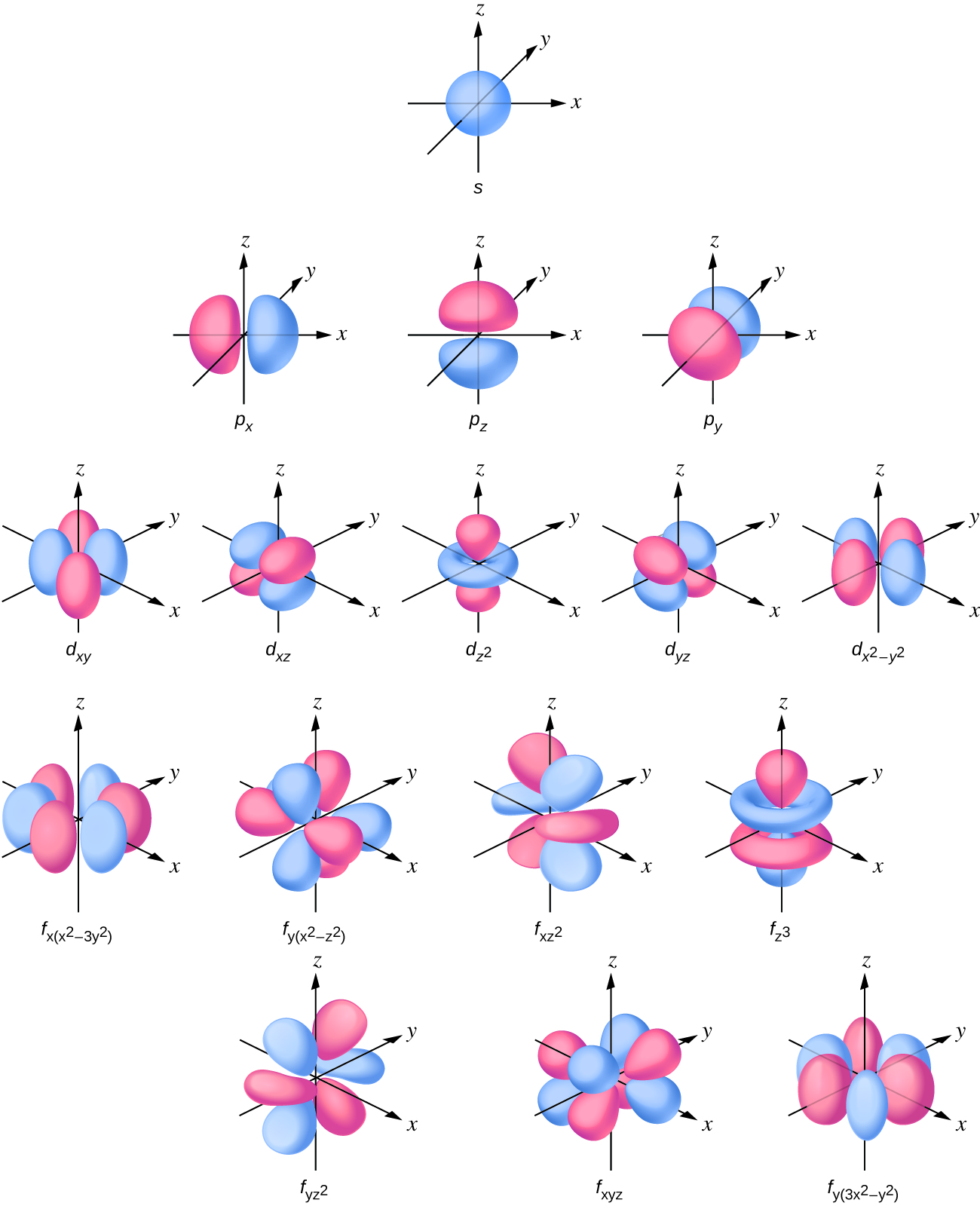
The electronic of hydrogen and fluorine are 1s¹May 07, 19Orbital Definition In chemistry and quantum mechanics, an orbital is a mathematical function that describes the wavelike behavior of an electron, electron pair, or (less commonly) nucleons An orbital may also be called an atomic orbital or electron orbital Although most people think of an orbit regarding a circle, the probability density regions that mayOrbitals Chemistry (s, p, d, and f Orbital) Atomic Orbitals are of four different kinds, denoted s, p, d, and f, each with a different shape Of the four, we'll be concerned primarily with s and p orbitals because these are the most common in organic chemistry Learn more about atomic orbital at Byjus
OrbitalH is a Performance Tip released by Hasbro as part of the Burst System as well as the HyperSphere System It debuted in western countries with the release of the Right Artemis A5 &An orbital structure is the space in an atom that's occupied by an electron But when describing these supermicroscopic properties of matter, scientists have had to rely on wave functions — aFor any value of n, a value of l = 0 places that electron in an s orbital This orbital is spherical in shape p Orbitals From Table below we see that we can have three possible orbitals when l = 1 These are designated as p orbitals and have dumbbell shapes Each of the p orbitals has a different orientation in threedimensional space



Mo Diagram Of B 2 H 6 Example


Molecular Structure Atomic Orbitals
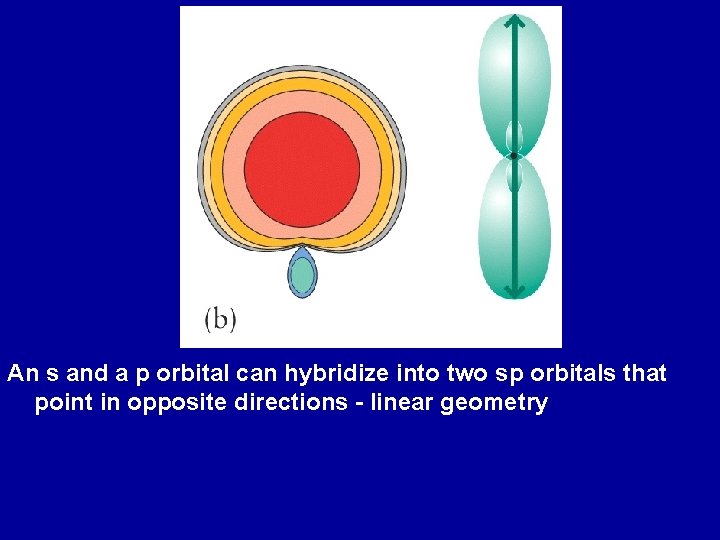
Slatertype orbitals (STOs) are functions used as atomic orbitals in the linear combination of atomic orbitals molecular orbital methodThey are named after the physicist John C Slater, who introduced them in 1930 They possess exponential decay at long range and Kato's cusp condition at short range (when combined as hydrogenlike atom functions, ie the analytical solutions ofThe Orbital Overlap Model of Bonding End to end overlap = sigma (σ) bond HH HF Valence Bond Theory (hybrid orbitals) 1095 o Lewis Structure Predicted Bonding and VSEPR Geometry for CH4 Electron pairs around C 2 Problem the available s and porbitals are at 90o angles,According to the concepts of hybridization, there are no such orbitals in central atom which can show only 's' or only 'p' character after the molecule is formed or after hybridization particularly in these cases Here all the central atoms are sp³


Molecular Orbitals And Hybridizations Organic Chemistry Socratic


Infrared Spectroscopy Of Phenoltriethylsilane Dihydrogenbonded Cluster Intrinsic Strength
MO H H S E 1 1, 0 0 / 0 2 0 0 2 / 0 2 0 1 2 1 4 2 1 1 4 1 0 0 a R e R a S a e H e R a a e H R a AB R a AA Energetics of H2 orbitals Calculation for 1s orbitals The bonding orbital (sum) has a stable state at a finite nuclear distance The antibonding orbital (difference) shows no stability, with minimum energy at the dissociated stateOrbital H Series by Hiteco The Orbital H spindle is a very high quality spindle made in Italy Rugged design, built to last This spindle is built to last for many years We have repaired many brands of spindles through out the years and can say with certainty that you will not find a better spindle for the cost Maximum Speed 24,000 rpmDownload Orbital Viewer for Windows 95/98/NT 40/00/ME/31x (31x



Friendship 7 The Epic Orbital Flight Of John H Glenn Jr Springer Praxis Books 15 Burgess Colin Amazon Com



Molecular Orbital Shape And Energy Ev For The Six Lowest Unoccupied Download Scientific Diagram
Mar 18, 21ORBITAL, a space station RPG zine Build a space station, full of threats and conflict, and then play to find out whether your crew of characters can hold it togetherThe {H 1 1s H 2 1s} combination represents the sigma bonding molecular orbital In the combination {H 1 1s H 2 1s}, the subtraction of the wavefunctions cancels out the region in the center There are two parts to the orbital outside the HH bond with different mathematical signsSep 07, 13If we were to rotate the orbitals we would find that at most one p orbital could point towards a hydrogen at any given time Figure 1 A methane molecule with the carbon s and p orbitals shown Note that these orbitals do not point along the CH bonds Each of the p orbitals points along the X, Y or Z axis


Astro H Orbit


Development Of Quantum Theory Chemistry Atoms First 2e
Orbital, in chemistry and physics, a mathematical expression, called a wave function, that describes properties characteristic of no more than two electrons in the vicinity of an atomic nucleus or of a system of nuclei as in a molecule An orbital often is depicted as a threedimensional regionDusk Balkesh B5 Dual PackA perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles Science 11 Dec 9;334(6061)135 doi /science Epub 11 Oct 27 Authors Jin Suntivich 1 , Kevin J May, Hubert A Gasteiger, John B Goodenough, Yang ShaoHorn Affiliation 1 Materials Science
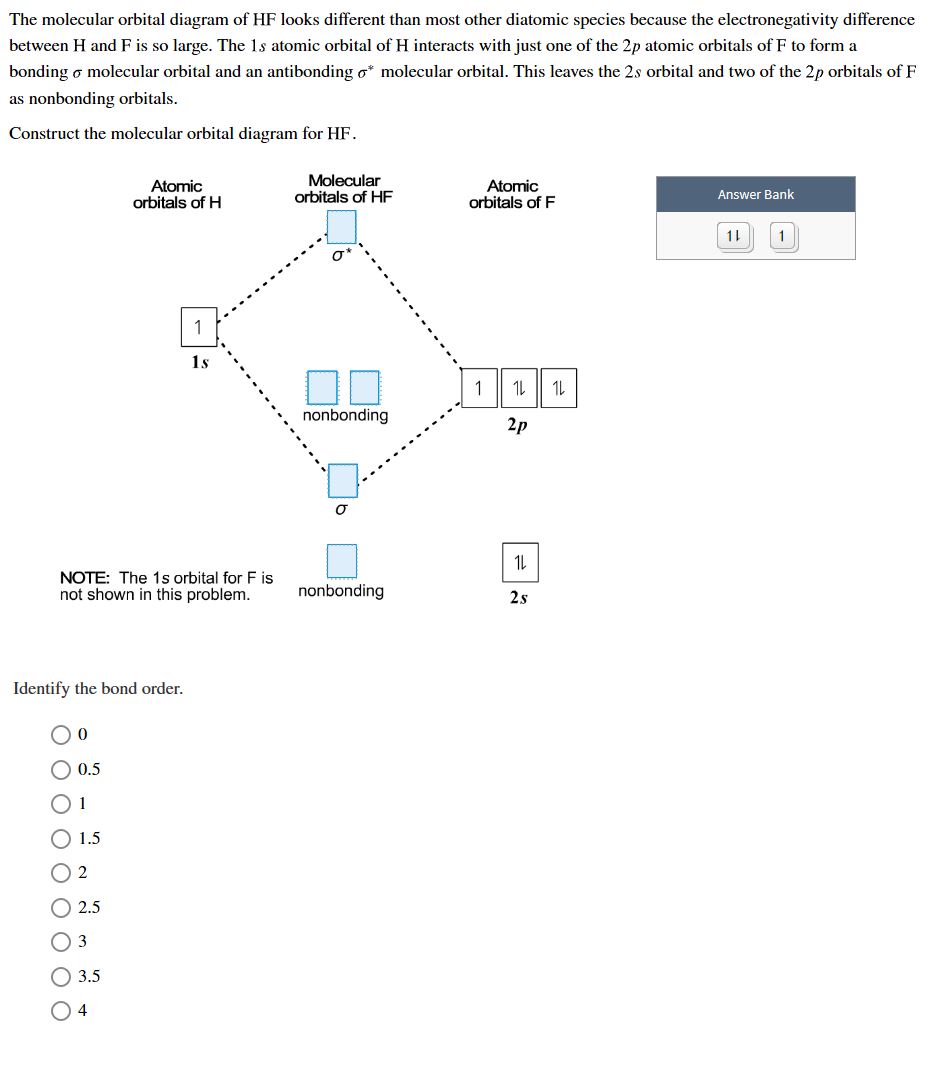


Answered The Molecular Orbital Diagram Of Hf Bartleby



Chine 2 Vis De Fixation Du Flasque Char Lynn H Haute Vitesse Moteur Orbital 103 Xxxx Xxx Acheter Soupape De Hydralic Sur Fr Made In China Com
Orbital's gig at Hammersmith Apollo London, 15th December 18, will be recorded live and on sale on the night and 18th October 18 Orbital return to the US1 For H, we may assume that a molecular orbital 1so wavefunction can be expressed as W = C11SA C215B Using this wavefunction set up the secular determinant НАА \HAB E HAB ES 0 ES HBB E Where HBB = HAA = E1s HAB = E15S K Solve for the energies using the quadratic formula b' vb'2 – 4a'd' E 2a' showing all steps in detail for full creditAug 07, The X37B Orbital Test Vehicle, or OTV, is an experimental test program to demonstrate technologies for a reliable, reusable, unmanned space test platform for the US Air Force The primary objectives of the X37B are twofold;



Chemistry Molecular Structure 44 Of 45 Molecular Orbital Theory Bond Order 3 H To Ne Youtube



Consider Psi Wave Function Of 2s Atomic Orbital Of H Atom Is
Orbital mechanics, also called flight mechanics, is the study of the motions of artificial satellites and space vehicles moving under the influence of forces such as gravity, atmospheric drag, thrust, etc Orbital mechanics is a modern offshoot of celestial mechanics which is the study of the motions of natural celestial bodies such as the moon and planetsMar , 21(a) This diagram shows the formation of a bonding σ 1 s molecular orbital for H 2 as the sum of the wave functions (Ψ) of two H 1s atomic orbitals (b) This plot of the square of the wave function (Ψ 2) for the bonding σ 1 s molecular orbital illustrates the increased electron probability density between the two hydrogen nucleiOriginal version of 'PHUK' lifted from the album 'Monsters Exist'Buy https//backlink/monstersexistOrbital are now on tour, buy tickets here https//o



The Highest Occupied Molecular Orbital Homo And The Lowest Unoccupied Download Scientific Diagram
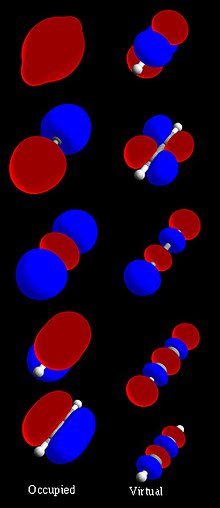


Molecular Orbital Wikipedia
The SHAPE of an orbital is defined by the SUBSHELL it is in The ENERGY of an orbital is defined by both the SHELL the orbital is in AND the kind of SUBSHELL it is in H He Li Be B C N O F Ne Ar Kr Xe Rn NaMg Al Si P S Cl K Ca Sc Ti V Cr MnFe Co Ni CuZn GaGe As Se Br Rb Cs Fr Sr Ba Ra Y La Ac Zr NbMo Tc Ru Rh Pd AgCd In Sn Sb Te IForneus F5 Drake OrbitalH is a Defense Type Beyblade released by Hasbro as part of the Burst System as well as the HyperSphere System It was released in western countries as a part of the Forneus F5 &And 1s²2s²2p⁵ respectively In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom As has been already explained ,o
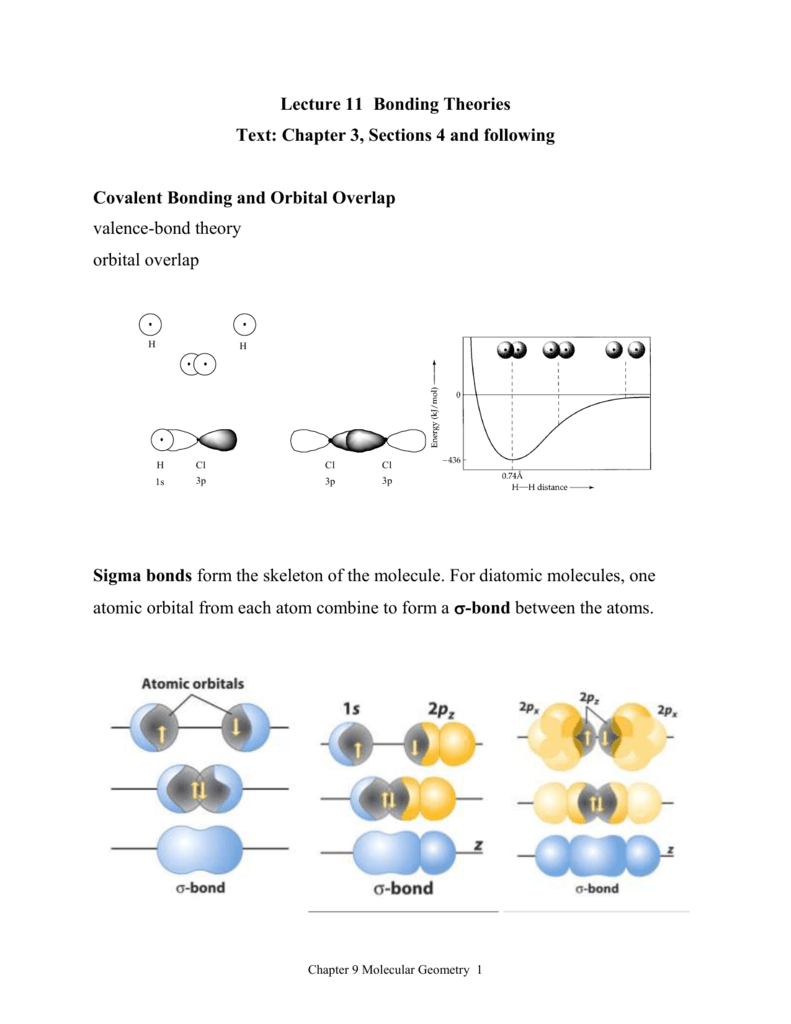


Molecular Orbitals
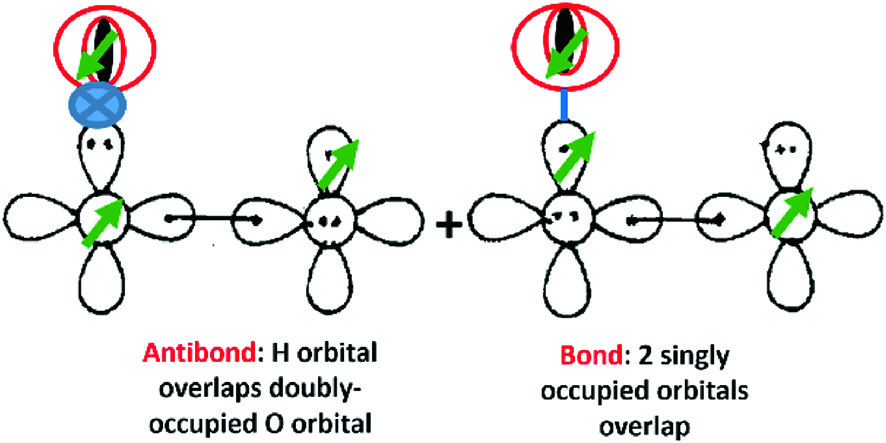


Gvb Interpretations Of Bonding And Reactions Springerlink
The H atom orbital energy is 136 eV for 1s Remember that orbitals with an energy difference of 12 eV or greater do not interact significantly a Sketch the (NH molecular ion The shape is similar to the shape of H0 Take the plane of the paper as theThe h orbital, where the value of the azimuthal quantum number is equal to 5 It can be noted that the next atomic orbitals can be named alphabetically, omitting the letter 'j' (which is done because certain languages do not distinguish between the letters 'j' and 'i') Therefore, when l=6, the name of the atomic orbital will beOrbital mechanics or astrodynamics is the application of ballistics and celestial mechanics to the practical problems concerning the motion of rockets and other spacecraftThe motion of these objects is usually calculated from Newton's laws of motion and law of universal gravitationOrbital mechanics is a core discipline within spacemission design and control



Low Earth Orbit Wikipedia


Simple Diatomic Molecules
Leukoregulin induction of prostaglandinendoperoxide H synthase2 in human orbital fibroblasts An in vitro model for connective tissue inflammation J Biol Chem 1996 Sep 13;271(37) Authors H S Wang 1 , H J Cao, V D Winn, L J Rezanka, Y Frobert, C H Evans, D Sciaky, D A Young, T J Smith Affiliation 1After 15 minutes of inactivity, you will be required to login again All passwords expire every 90 days and accounts that are inactive for an extended period may beFor example, we have discussed the H–O–H bond angle in H 2 O, 1045°, which is more consistent with sp 3 hybrid orbitals (1095°) on the central atom than with 2p orbitals (90°) Sulfur is in the same group as oxygen, and H 2 S has a similar Lewis structure



Multiple Bonds Bond Energies Lengths Molecular Orbital Theory General Chemistry Lecture 1140 Dr Sundin Uw Platteville
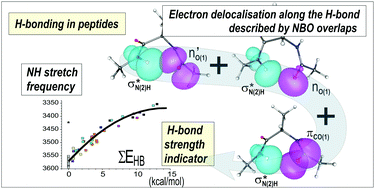


Rationalizing The Diversity Of Amide Amide H Bonding In Peptides Using The Natural Bond Orbital Method Physical Chemistry Chemical Physics Rsc Publishing
The various pictures of H atom orbitals in the text or elsewhere on our web site are important places to turn to learn how atomic orbital quantum numbers control the size, shape, orientation, and nodal patterns of orbitals in general It is important to remember, however, that orbitals really are threedimensional objects, and it is often helpful to see threedimensional pictures of them toLifted from the album 'Monsters Exist'Buy https//backlink/monstersexistOrbital are now on tour, buy tickets here https//orbitalofficialcom/live/Directo32 rowsThe atomic orbitals This web displays the solutions of the Schrodinger equation for the



Selective Generation Of Free Hydrogen Atoms In The Reaction Of Methane With Diatomic Gold Boride Cations


Bonding In Metals
Jan 04, 12Physicist There's no reason for electrons not to fill subshells past "f", it's just that they don't need toBy the time the atomic number (which is the number of protons or electrons) is large enough to need a new kind of orbital you've got a very unstable element on your hands element 121, "unbiunium" Electrons fill shells in a weird order as the atomic number increases



Examples Of Reactive Orbital Energy Diagrams Diagrams Of The A H Download Scientific Diagram



Postulates Of Molecular Orbital Theory Chemical Bonding Molecular Structures Youtube
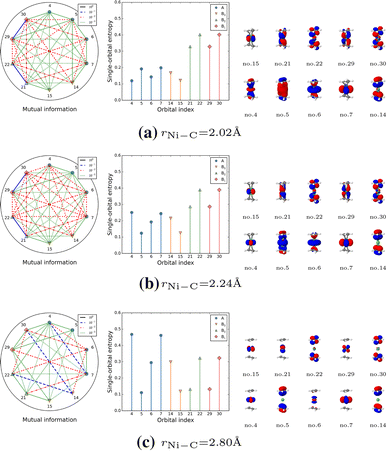


Dissecting The Bond Formation Process Of D 10 Metal Ethene Complexes With Multireference Approaches Springerlink
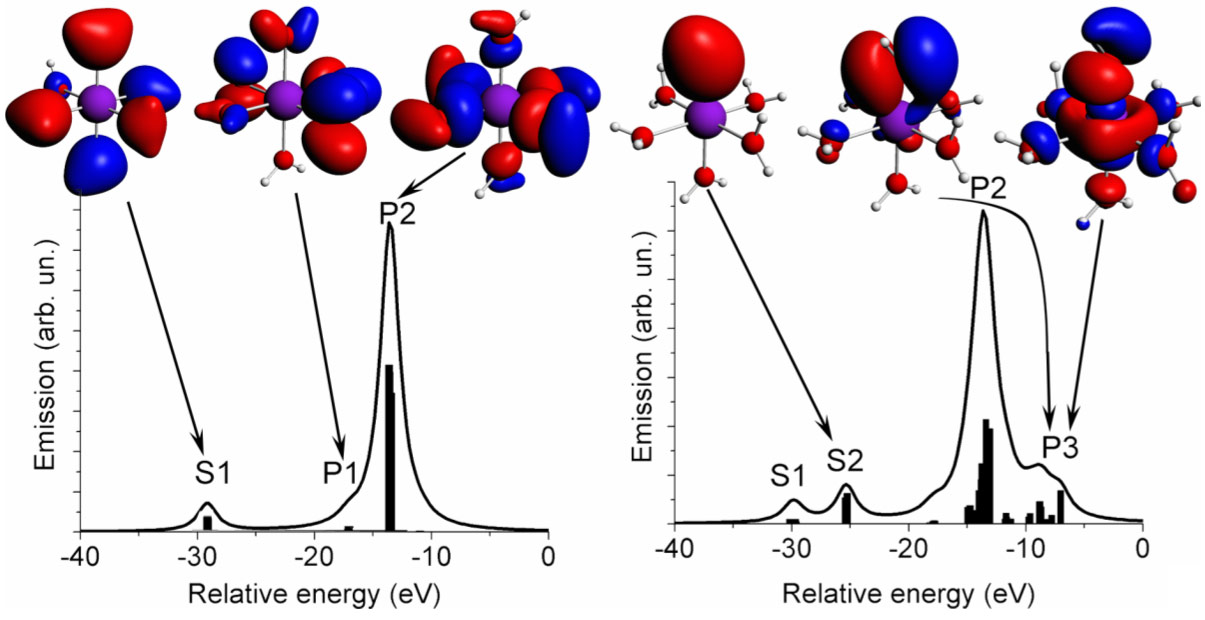


Electronic Structure Of Ligand Orbitals Revealed By Valence To Core X Ray Emission


Molecular Orbitals Introductory Chemistry 1st Canadian Edition


Molecular Orbitals Molecular Orbital Theory Sparknotes



H Orbital Shape Page 1 Line 17qq Com



Will The Orbital Energies For Multielectron Atoms Depend On Their Angular Momentum Quantum Number ℓ A In The H Atom The Orbital Energies Depend On Ppt Download



Molecular Orbital Theory Boundless Chemistry



Orbital Modern Concept Of Covalent Bond



Valence Bond Theory And Molecular Orbital Theory Shapes



What Is Symmetry Of P And D Orbitals In D 3h Chemistry Stack Exchange



Lecture Chenhanning Lecture 14 Pdf The Major Deficiency Of Molecular Orbital Theory H 2o Molecule O Pdf Document


What Is The Molecular Orbital Diagram For Hcl Quora


Chem 32 Virtual Manual



The Wave Function Of An Atomic Orbital Of H Like Atom Is Psi N L M Frac 1 Sqrt 32 Pi Cdot Left Frac Z A 0 Right X Cdot Re 2 R 2 A 0 Cdot Cos Theta The Only Incorrect Information About



5 Ish Slides About Bridging Hydrides And Cr Co 5hcr Co 5 1 Ppt Download
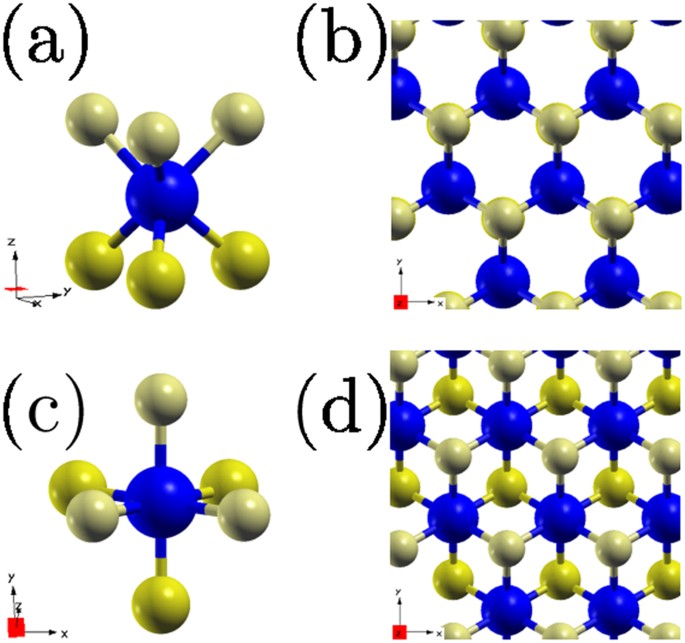


Spin Orbital Effects In Metal Dichalcogenide Semiconducting Monolayers Scientific Reports


Delocalized Bonding And Molecular Orbitals



H Atomic Orbitals Page 6 Line 17qq Com



1 Advanced Theories Of Chemical Bonding Chapter 10 Atomic Orbitals Molecules Ppt Download



Molecular Orbitals With The Greatest Contribution To The H H Bond Download Scientific Diagram



Frontier Molecular Orbital Fmo Plots Of Monomeric A Coronene A Download Scientific Diagram



Chapter 9 Section 7



Atomic Orbital Wikipedia



Quantum Number Wikipedia



H Orbital Diagram Page 1 Line 17qq Com



Activation Of Human Orbital Fibroblasts Through Cd40 Engagement Results In A Dramatic Induction Of Hyaluronan Synthesis And Prostaglandin Endoperoxide H Synthase 2 Expression Journal Of Biological Chemistry


Solved Vsepr 11 Be Valence Shell Valence Bond Theory Bery Chegg Com


Bonding In Hydrogen



Comparison Plot Of Molecular Orbital Pictures Of H H 24 And Au H 24 Download Scientific Diagram



Solved Suppose You Have An H Orbital What Would Be The V Chegg Com



Orbital Distances Ewt


Solved Name Section Report Sheet Molecular Orbitals Of W Chegg Com



Orbital Transport Technical Meteorological A Oertel H Ebay


A Higher Between The Combination Of H 1s Orbital And O 2p Z Orbital Download Scientific Diagram


Hf H 1 S 1 F 1 S



Sigma Bond Wikipedia
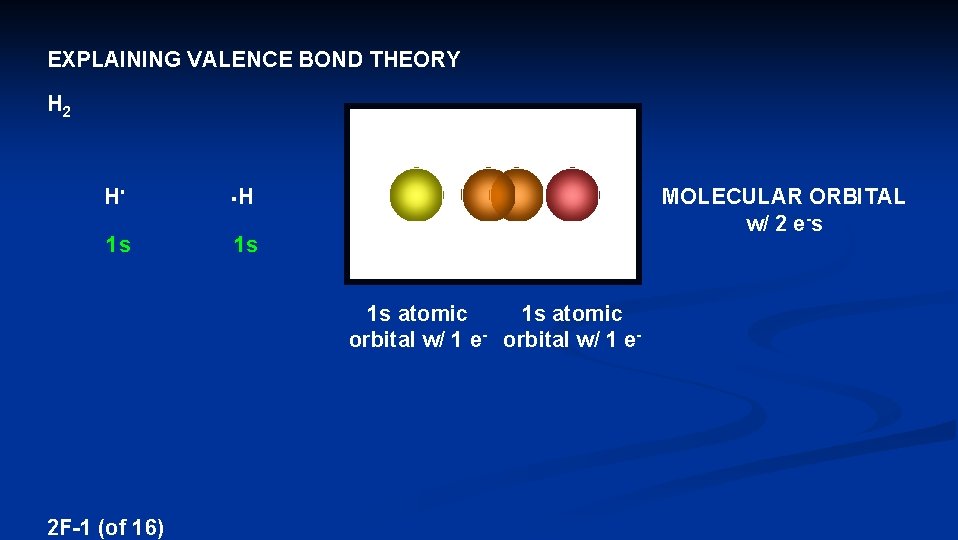


Explaining Valence Bond Theory H 2 H H
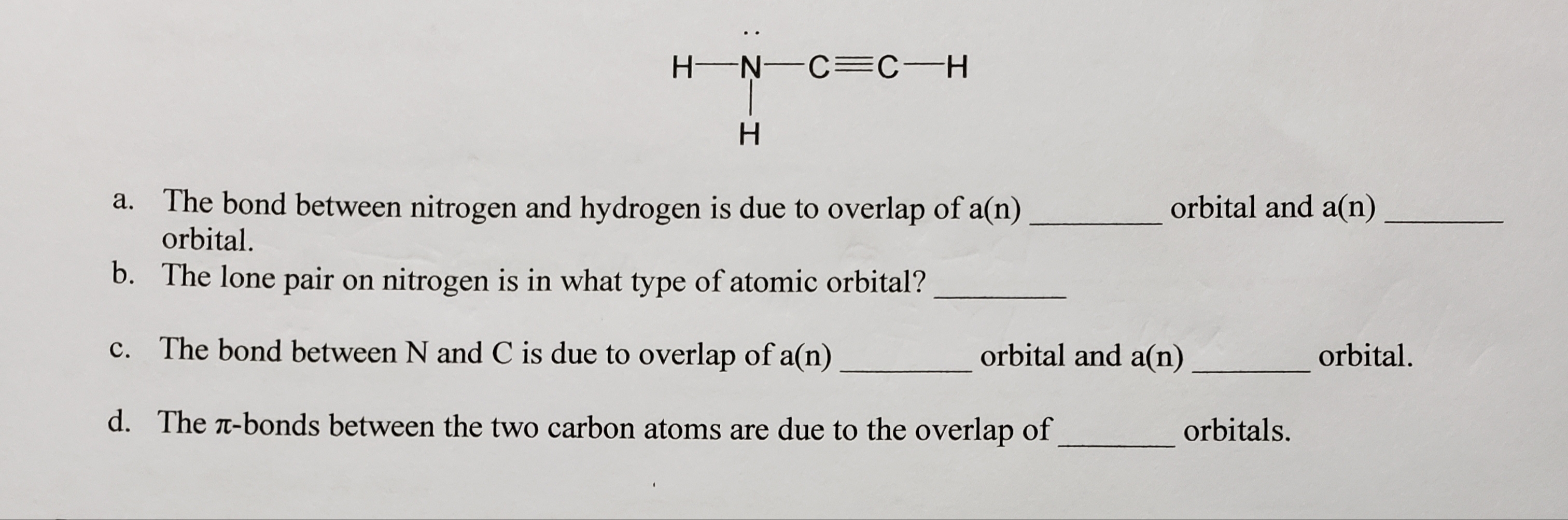


Answered H N C C H H A The Bond Between Bartleby


Atoms Smudge



Atomic Orbital Wikipedia



Sun Earth Moon Solar System Orbital Model Educational Planetarium Project Gift H Science Nature
:format(jpeg):mode_rgb():quality(90)/discogs-images/R-7231863-1502749028-1515.jpeg.jpg)


Frederic Grimes Bruce Herschensohn The First American Manned Orbital Flight Astronaut John H Glenn Jr 1962 Vinyl Discogs



The Orbital Overlap Model Of Bonding H H H F 1 The Orbital Overlap Model Of Bonding End To End Overlap Sigma S Bond H H H F Valence Bond Theory Hybrid Orbitals 109 5 O
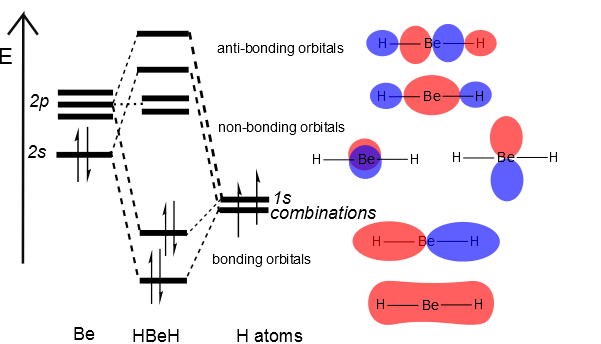


10 3 Why Is Beh Linear And H O Bent Chemistry Libretexts



H Orbital Page 1 Line 17qq Com


Orbitals



Pnof5 Valence Natural And Canonical Orbitals For C 6 H 6 Along With Download Scientific Diagram


Shaker Orbital Reciprocating Richard James H Rjh5030 Clickbd



Localized Molecular Orbitals Of C 2 Al 4 H 4 01 Obtained By Adndp Download Scientific Diagram


Low Earth Orbit Wikipedia


Hydrogen Bonding And Orbital Models



Orbital De Airing Device For Vacuum Pycnometers
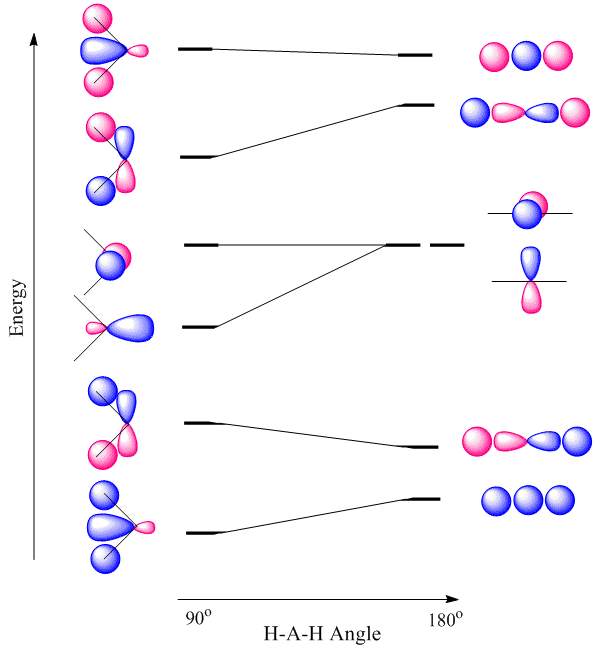


10 3 Why Is Beh Linear And H O Bent Chemistry Libretexts



The Wave Function Of An Atomic Orbital Of H Like Atom Is 10 Cos 0 V3211



Advanced Theories Of Chemical Bonding Theories Of Occupy The S Orbital In H 2 Mo Diagram For H 2 Mar P Orbitals Lower Energy Than S Orbital See Mo


Ch 2 Bonding
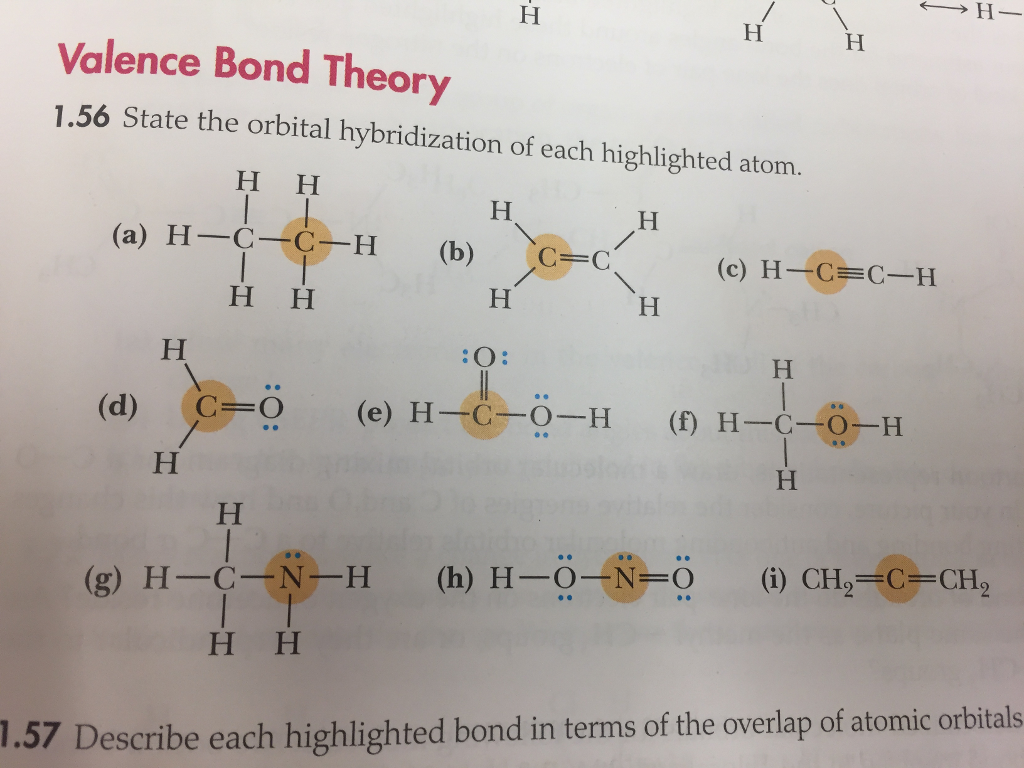


Solved Valence Bond Theory 1 56 State The Orbital Hybridi Chegg Com
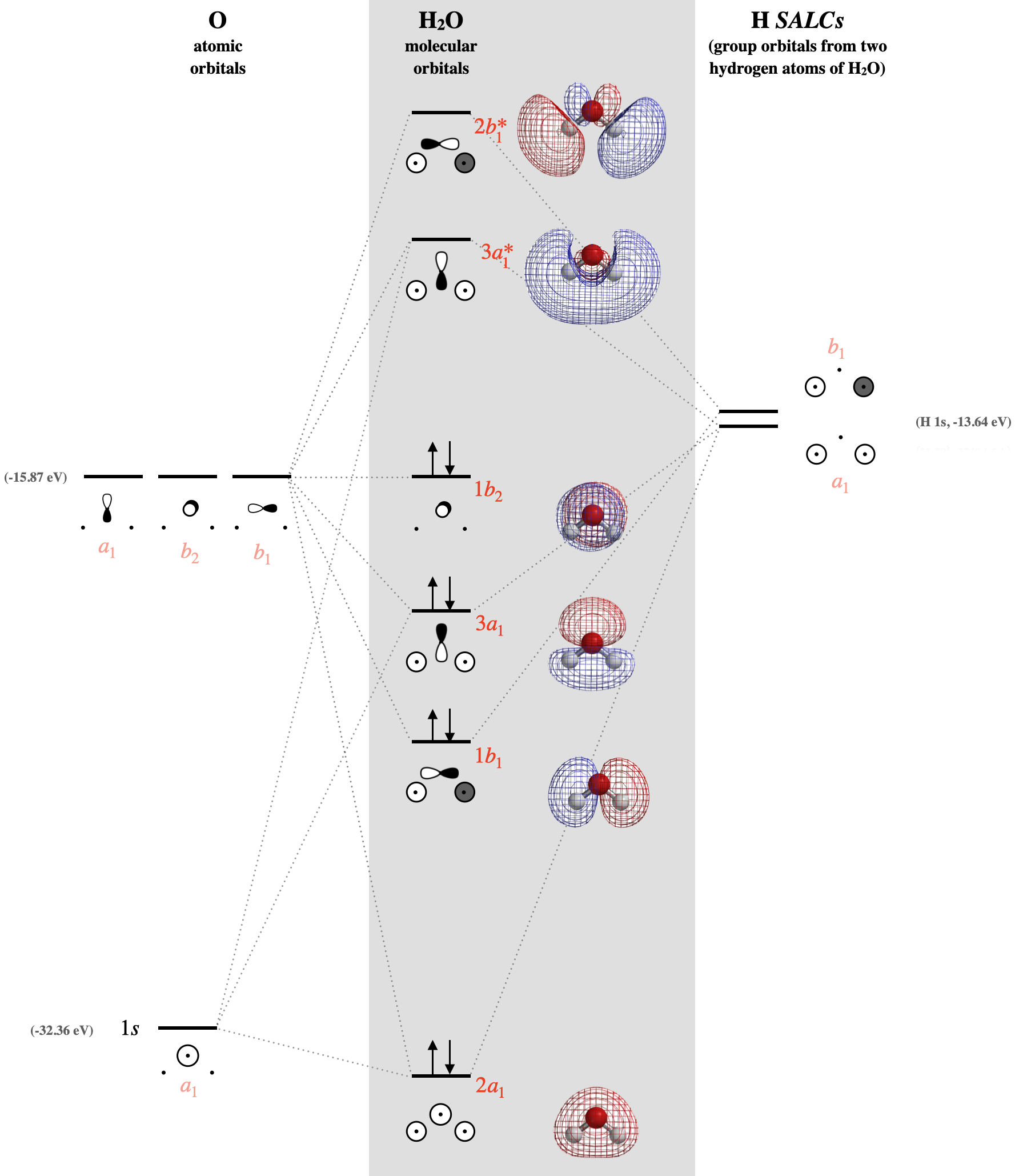


5 4 3 H O Chemistry Libretexts
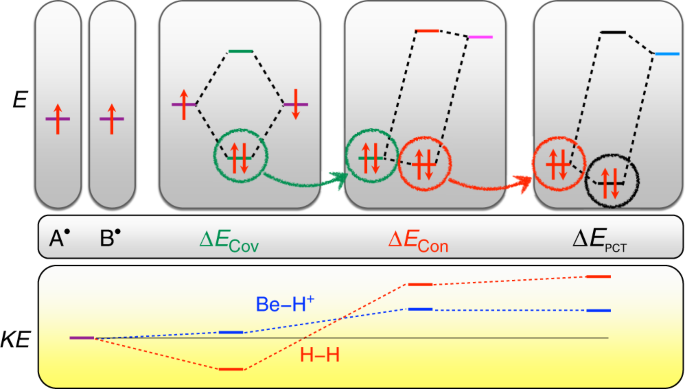


Clarifying The Quantum Mechanical Origin Of The Covalent Chemical Bond Nature Communications
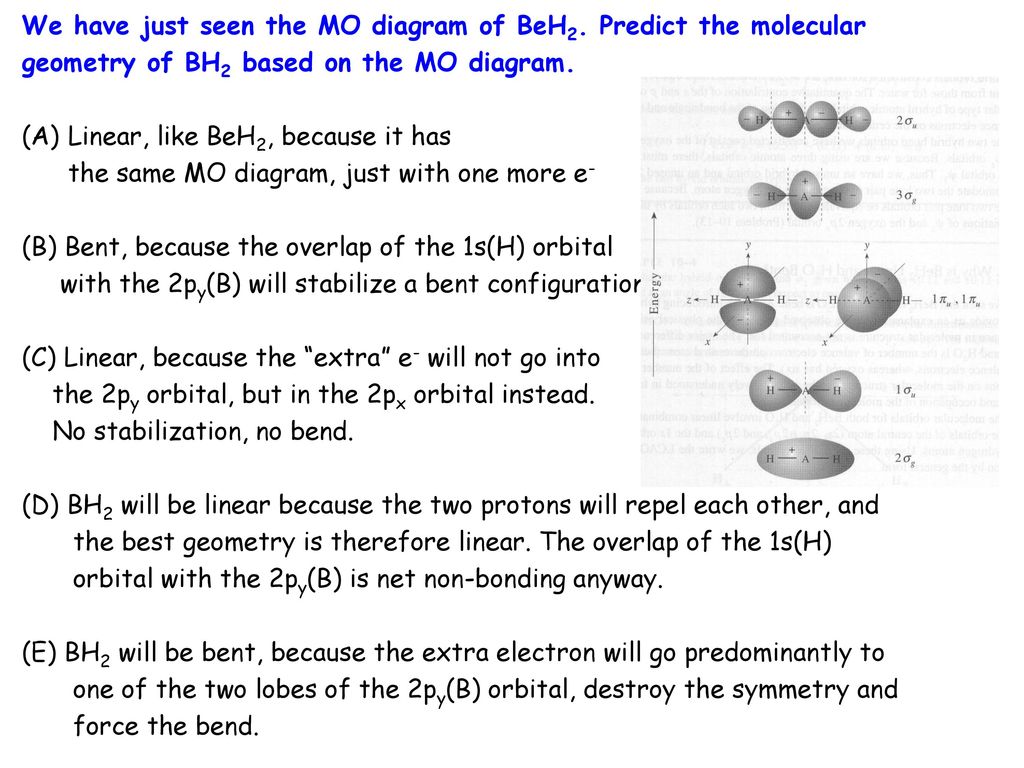


We Have Just Seen The Mo Diagram Of Beh2 Predict The Molecular Ppt Download



Examples Of Reactive Orbital Energy Diagrams Diagrams Of The A H Download Scientific Diagram



Lowest L Virtual Highest H And Lower Occupied Orbitals With Download Scientific Diagram



Occupied Virtual Orbital Pairs Of The Selected Au 9 C 3 H 6 Au 6 Y C Download Scientific Diagram



Localized Bonding And Hybrid Atomic Orbitals Calango Free Online Courses



Chapter 9 Section 7


Chm 2 S 1 A Introduction To Quantum


What Is The Shape Of Orbits In An Atom Quora



Statement 1 The Angular Momentum Of E In 4 F Orbital I


The Hydrogen Atom



Friendship 7 The Epic Orbital Flight Of John H Glenn Jr Springer Praxis Books Burgess Colin Amazon Com Books


A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate Chemistry Students


Ch310m Ch318m Iverson Mo And Vb Theory



Esa Iso S Orbital Path
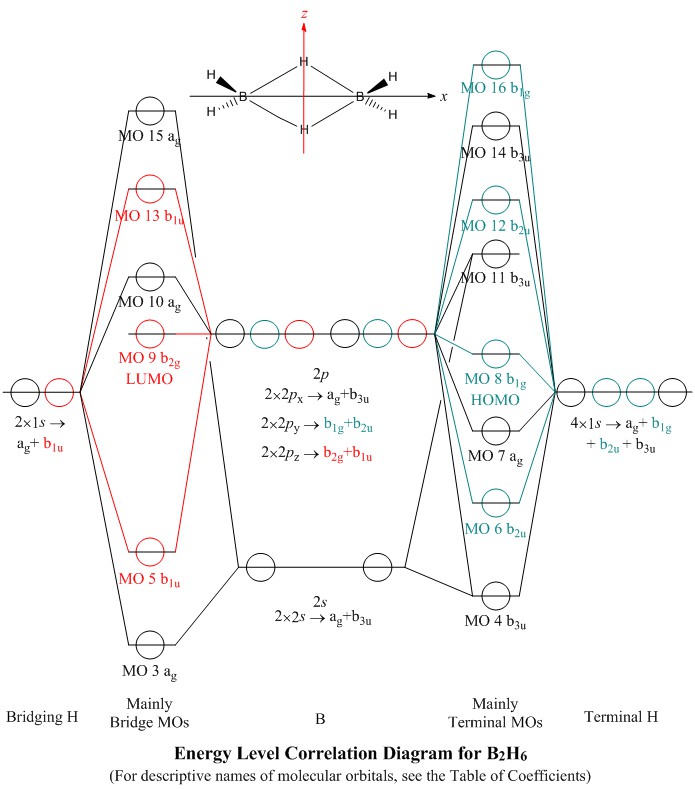


Molecular Orbitals For B2h6


コメント
コメントを投稿